Pharmacovigilance
Our proactive approach enhances safety risk control
In a time of unprecedented acceleration in clinical development and ever-changing regulatory landscape, Pivotal will keep you in control of your drug safety matters. Our proactive approach allows us to get ahead of safety problems and maintain trial integrity and a lower risk profile. We use the latest safety technology and leverage our near real time analytics platform, Danah, to identify safety trends to enhance risk control.
Our staff are highly skilled MDs or hold an advance degree in life sciences, and they are fully versed in the regulatory safety requierements and with deep therapeutic expertise.
Our safety services
Using cutting-edge technology to monitor safety trends, ensuring your trial maintains integrity and a lower risk profile.
Related Insights
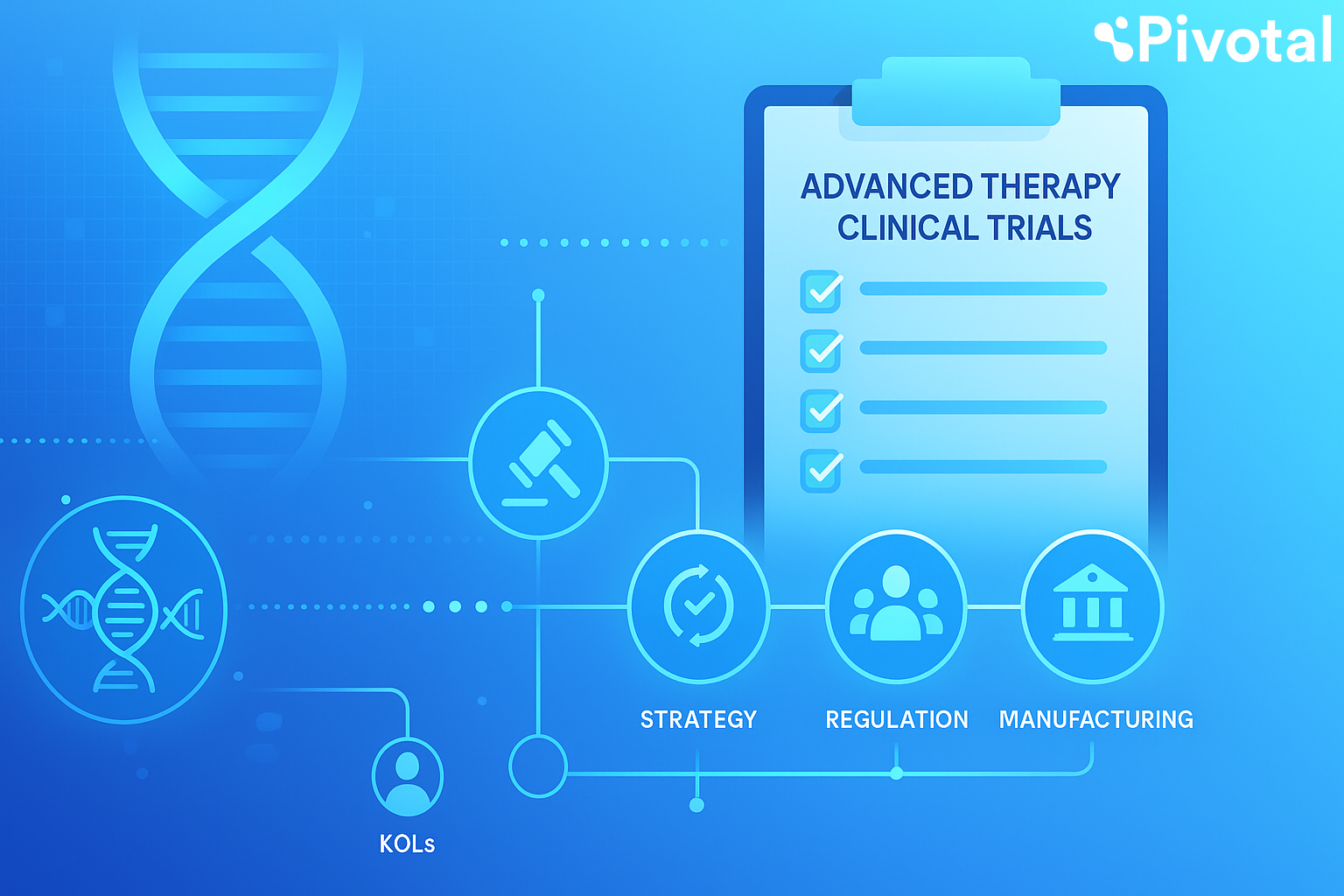
Our Solutions for Biotechs
We offer end-to-end clinical development solutions tailored to your needs. Whether you’re preparing for first-in-human or managing a global Phase 3 trial, our agile team delivers expertise, insight, and hands-on support at every stage.