
The Best Solution for Biotechs
Our insights solutions empower data scientists and medical monitors to ensure data quality and key value dimensions are consistently met.
With seamless risk identification, visualization, management, and documentation, our RBQM solution equips study teams with the tools they need to run smarter, faster, and more compliant trials.
Danah RBQM,
Site Profile & Insights
Modern trials with digital patient tools or with adaptive or decentralized designs, require flexible data collection that adapts to interim results and patient stratification. Beyond accuracy, data must also reflect the patient’s journey. With a risk-based approach, Danah helps explore, assess, and challenge clinical data through its modules.
Operational Insights
Explore the recruitment process with real-time KRI monitoring—track recruitment rates, early terminations, screen failures, and much more. Stay on top of site performance with our follow-up items and deviations tracker for seamless oversight.
Clinical Insights
Key data in your trial such as adverse events and laboratory tests displayed as aggregated tables, listings and graphs including monitoring of key lab values throughout the trial for a specific patient vs study population.
Patients Profiles
Effortlessly access comprehensive patient data directly from our platform, including disease assessments, dosing schedules, and adverse events.
Central Statistical Monitoring
By analyzing trends and applying statistical tests, gain deeper insights into patient progress with our Central Statistical Monitoring, featuring reports on adverse event rates, lab value trends, top medical history terms, and more.
Key Risks Indicators (KRIs)
Monitor sites performance utilizing our KRIs for safety, queries lifetime, missing data, etc. Explore our library of KRIs aligned with Transccelerate recommendations or let us create specific metrics for your trial.
Quality Tolerance Limits
We apply statistical tests to determine which thresholds shall be set for sites and KRIs or utilize our expertise to pre-define them and be aware of any signal arising from our Danah RBQM module.
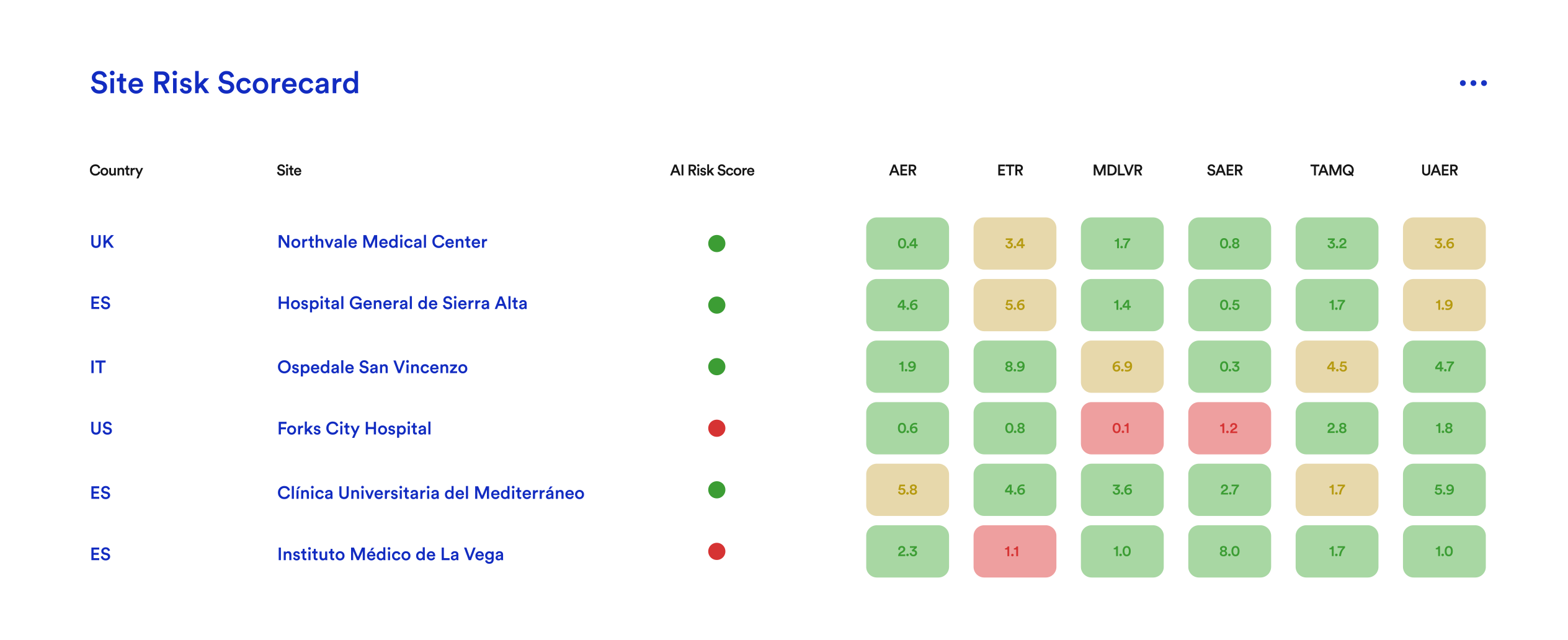
Site Risk Scorecard
KRIs are essential for reporting site performance, but identifying truly underperforming sites requires more. That is where Danah helps—our machine learning classifier analyzes key risk factors to flag sites needing attention, enabling proactive management and improved trial quality.
Risk assessment tracker
Track all study risks and assessments and define the path to control them. Automatically publish all versions of Danah’s Risk Tracker.
Signal and action tracker
This tool continuously monitors signals and triggers alerts when performance issues or data inconsistencies arise. With an intuitive interface, it helps you prioritize and manage corrective actions, ensuring timely interventions to mitigate risks and keep your clinical trial on track.
Danah
AI eTMF
Automate your document management with Danah’s AI-powered eTMF. Our system automatically reads and classifies documents, assigns metadata, and ensures they are correctly uploaded, reducing administrative burdens and ensuring inspection readiness. Danah’s Working Area allows study teams to upload, edit, review, e-Sign and QC documents seamlessly and within the system.
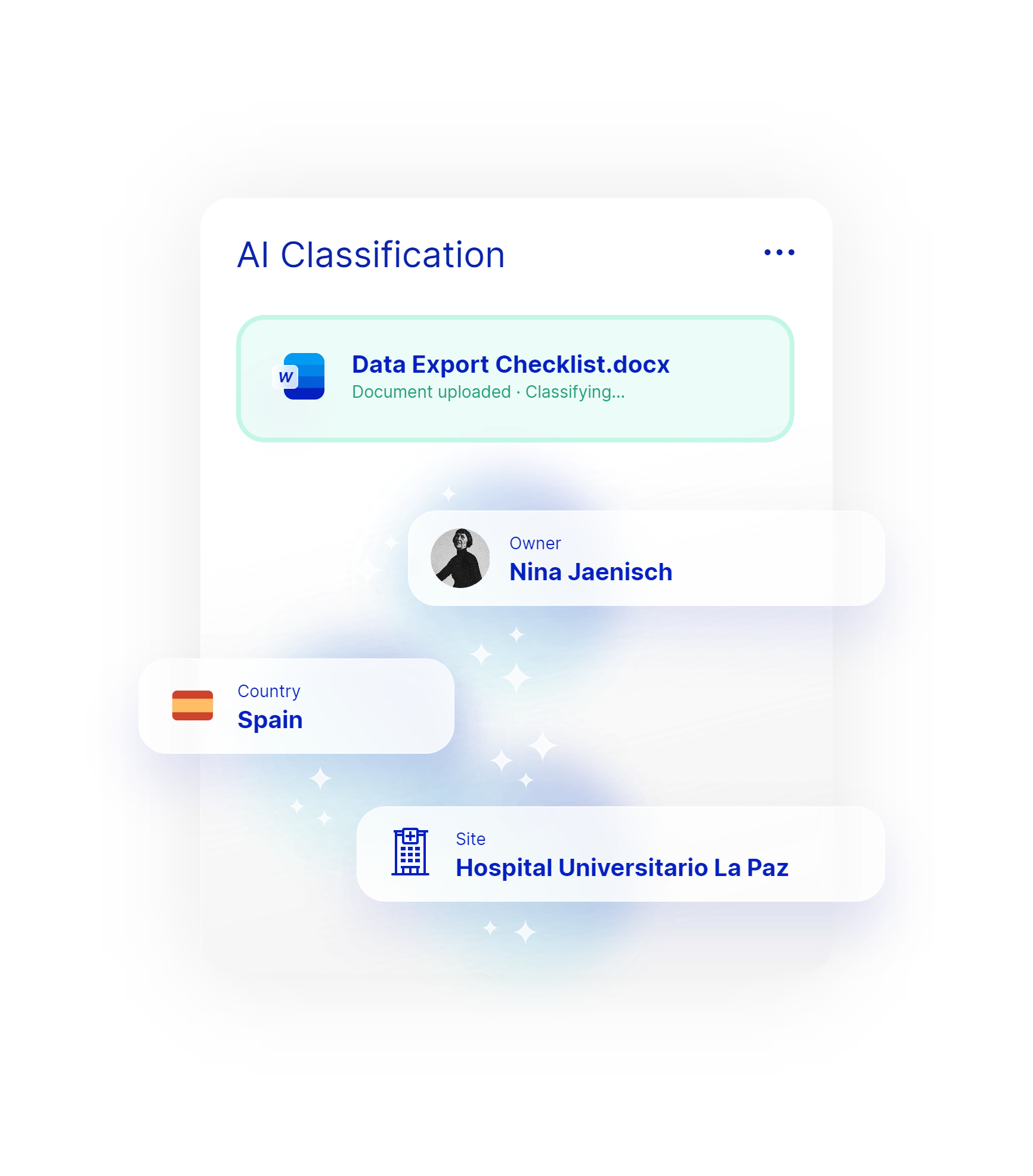
Danah
CTMS & Training
Danah’s e-CTMS integrates all aspects of clinical trial management, from study planning and tracking to monitoring and training. Enjoy a 30% reduction in monitoring, planning and training time with our end-to-end study management capabilities. Track study timelines, resource allocation, and operational performance in real-time to enhance trial efficiency.
Ensure your study team is always up-to-date with the latest regulations and quality standards. Danah’s Study Training module allows you to create and manage training programs, assign courses, track progress in real-time, and generate certificates of completion for your team members.
